Paz Lab
Neurological Disease
Network Dynamics and Plasticity in Epilepsy
Areas of investigation_
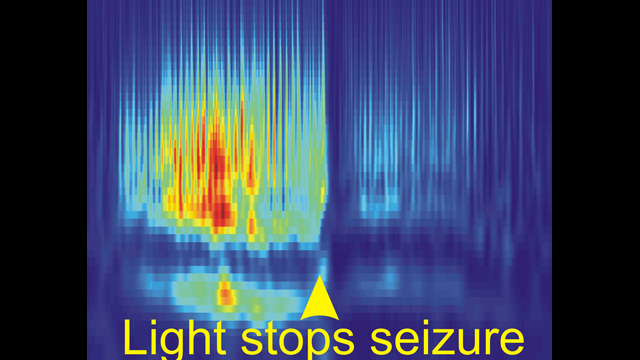
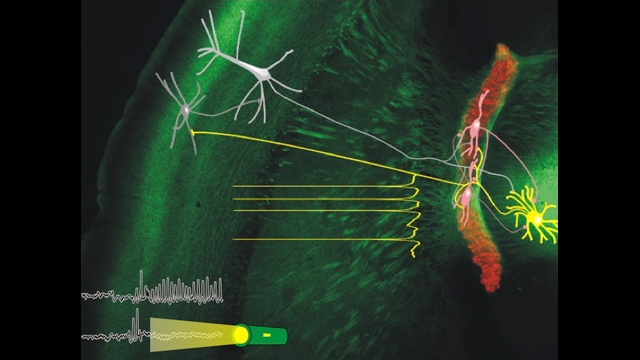
Our lab focuses on studying how neural synchronization and plasticity relate to adaptive and maladaptive behavior. Our interests span many levels of analysis, from the cell to the circuit to animal behavior. For example, in animal models, we disrupt neural plasticity and normal circuit dynamics to learn about how these functions recover after brain injury. We also dissect the cellular, circuit, and molecular mechanisms by which brain injuries, cerebrovascular disease, and genetic mutations cause neurological disorders such as epilepsy. Most notably, we are using optogenetic tools, which allow the control of specific elements of intact biological systems using light, to interrogate cells and synaptic components involved in neural circuit oscillations (i.e. epileptic seizure). We then couple these results with our in vitro findings to determine the cellular and microcircuit mechanisms that relate to these oscillations. After we identify the neural circuit that alleviates symptoms, we then target these circuits in the behaving animal at the onset of abnormal brain activity in real-time. The goal of this approach is to stop seizures before they start.
Significance
Epilepsy develops in 1 out of 26 people during the course of their life and can be caused by genetic mutations, brain injuries, tumors, and some infectious diseases. For example, the incidence of epilepsy is high in soldiers suffering traumatic brain injury and is increasing in the aging population afflicted by Alzheimer’s disease, strokes, and tumors. Despite these correlations, the cause of this condition is unknown in more than half of patients suffering from epilepsy. Many antiepileptic drugs exist, but they often have debilitating side effects and do not fully suppress the highly disruptive and potentially fatal symptoms in patients. Notably, half of the three million Americans with epilepsy cannot completely control their seizures with treatment, or only do so at the cost of debilitating side effects. Thus, we need to discover new therapeutic strategies that effectively treat epilepsy.
Therapeutic interest
Our ultimate goal is to discover therapeutic targets that lead to effective therapies for epilepsy. To work toward this goal, we are improving our knowledge of the pathophysiological mechanisms that cause neuronal synchrony in epilepsy by investigating how epileptic activity is initiated and maintained. More specifically, we are using optogenetic tools to identify targets that alleviate symptoms of epilepsy without causing side effects. Our work will provide a foundation on which researchers can develop novel treatments for seizures that occur in epilepsy and other neurological or cognitive disorders.
Approaches
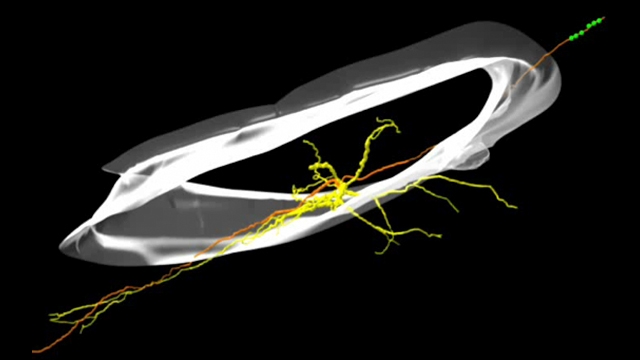
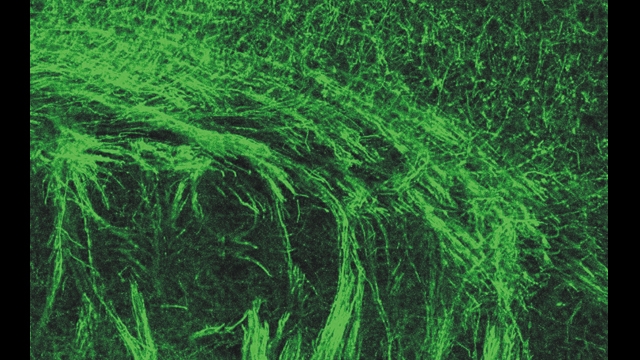
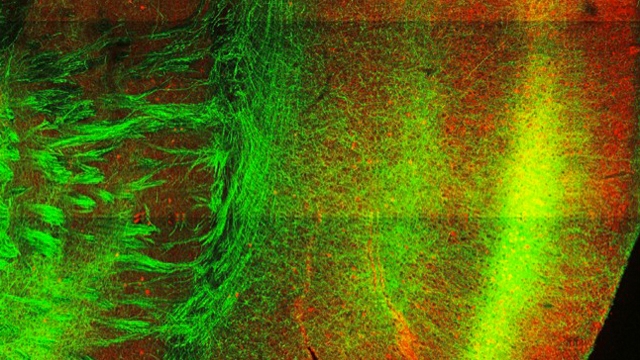
We are using a multidisciplinary approach in genetic and pharmacological animals models of human disease, including, 1) a combination of optogenetic tools to manipulate cells in vivo and in vitro; 2) intracellular electrophysiology in rodents and whole-cell patch-clamp electrophysiology in brain slices to dissect single-synaptic and large-scale network activity; 3) laser scanning/glutamate uncaging in brain slices to map circuit rewiring; 4) pharmacology to study the molecular mechanisms of anti-epileptic drugs; 5) programmable processors to detect seizures and control these by closed-loop optogenetic approaches in real-time in behaving rodents; and 6) complementary neuroanatomy to track the morphology of neurons.
Contributions
Our recent publication (Paz et al., Nature Neuroscience 2012) was the first to reveal that seizures can be instantaneously aborted in real-time with closed-loop optogenetic control of a specific cell type. This work led us to identify thalamocortical neurons as novel targets that control post-stroke seizures in real-time without side effects. We believe that this same approach will reveal control points in the brain—regions, cells, and circuits—in other forms of epilepsy and neurological and cognitive disorders.
Some questions addressed in ongoing and future studies
- Which key cells and synaptic targets are sufficient to control seizures in real-time without side effects?
- What forms of neural plasticity affect the recovery of brain function after injury and in cerebrovascular diseases?
- What are the mechanisms that cause epilepsy after brain injuries such as stroke?
- How can we prevent epilepsy from developing after brain injury?
- What are the mechanisms that underlie seizure initiation, spread and modulation?
- What mechanisms underlie loss of consciousness during seizures?
- What are the co-morbidities of network dysfunction in epilepsy and psychiatric and cognitive disorders (e.g., Alzheimer’s disease, schizophrenia, autism)?

 Associate Investigator,
Associate Investigator,